is f2 polar or nonpolar F2 lewis dot structure
Hey everyone, let’s dive into the fascinating world of chemistry today! Today, we will be discussing the polarity of Fluorine (F2) and its Lewis dot structure. So, buckle up and get ready for some interesting insights!
F2 (Fluorine) - Polar or Nonpolar?
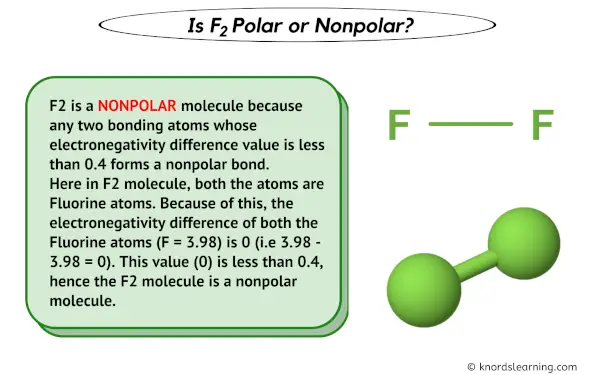
 First things first, let’s determine whether Fluorine (F2) is polar or nonpolar. When it comes to molecular polarity, it depends on the electronegativity difference between the atoms involved. In the case of Fluorine, it is a diatomic molecule, meaning it exists as F2 in its elemental form.
First things first, let’s determine whether Fluorine (F2) is polar or nonpolar. When it comes to molecular polarity, it depends on the electronegativity difference between the atoms involved. In the case of Fluorine, it is a diatomic molecule, meaning it exists as F2 in its elemental form.
Fluorine is the most electronegative element on the periodic table, which means it has a strong ability to attract shared electrons towards itself. Since both the atoms in F2 are identical and have the same electronegativity, there is no significant electronegativity difference, making it a nonpolar molecule.
F2 Lewis Dot Structure
 To understand the bonding in Fluorine (F2), we can analyze its Lewis dot structure. The Lewis dot structure is a diagrammatic representation of the valence electrons in a molecule, where dots indicate the valence electrons of an atom.
To understand the bonding in Fluorine (F2), we can analyze its Lewis dot structure. The Lewis dot structure is a diagrammatic representation of the valence electrons in a molecule, where dots indicate the valence electrons of an atom.
In the case of F2, each Fluorine atom has seven valence electrons. By following the octet rule, where atoms tend to gain, lose, or share electrons to achieve a stable configuration of eight electrons, we can represent the Lewis dot structure of F2.
As shown in the image above, the two Fluorine atoms share a single bond by sharing one electron each. This bond is represented by a shared pair of electrons between the two F atoms. Both Fluorine atoms now have eight electrons in their outermost shell, fulfilling the octet rule.
It is important to note that the Lewis dot structure of F2 does not show any polarity because the electrons are equally shared between the two atoms, resulting in a nonpolar molecule.
In conclusion, Fluorine (F2) is a nonpolar molecule, and its Lewis dot structure consists of a single bond formed by sharing a pair of electrons between the two Fluorine atoms. Understanding the polarity and structure of molecules is essential in the field of chemistry as it affects the physical and chemical properties of substances.
I hope this short journey into the world of Fluorine has piqued your interest in chemistry. Stay curious and keep exploring!
If you are searching about Is F2 ( Fluorine ) polar or nonpolar you’ve visit to the right page. We have 5 Pictures about Is F2 ( Fluorine ) polar or nonpolar like is f2 polar Gallery, Is F2 Polar or Non-polar? (Fluorine Gas) - YouTube and also is f2 polar Gallery. Read more:
Is F2 ( Fluorine ) Polar Or Nonpolar
 www.bengislife.comf2 polar nonpolar non fluorine polarity
www.bengislife.comf2 polar nonpolar non fluorine polarity
F2 Lewis Dot Structure
 aunitedkingdomfilm.comIs F2 Polar Or Nonpolar? (Why? & How?)
aunitedkingdomfilm.comIs F2 Polar Or Nonpolar? (Why? & How?)
 knordslearning.comIs F2 Polar Gallery
knordslearning.comIs F2 Polar Gallery
 keywordteam.netIs F2 Polar Or Non-polar? (Fluorine Gas) - YouTube
keywordteam.netIs F2 Polar Or Non-polar? (Fluorine Gas) - YouTube
 www.youtube.comf2 polar fluorine gas non
www.youtube.comf2 polar fluorine gas non
Is f2 ( fluorine ) polar or nonpolar. Is f2 polar or non-polar? (fluorine gas). F2 polar fluorine gas non